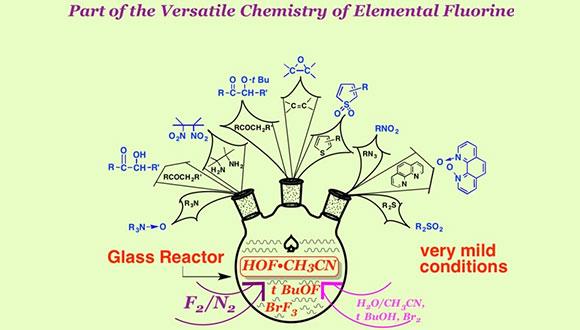
Fluoro-Organic Chemistry
From the time of creation till the mid twentieth century Nature did not bother to create more than two dozen fluorine containing products (almost all toxic), despite the fact that this element is among the most abundant in the earth crust (e.g. more than chlorine). The 20th century, however, gave the signal for a huge revolution in the field, a revolution that still is in full swing till today.
Thus there is not a single area of our life which is not profoundly affected by fluorine. Let us take, for example, air conditioning and refrigeration which rely on small fluorinated molecules (old and new Freons). We could look at the most stable fluoropolymers which give us the opportunity to use organic materials in extreme conditions (see for example space exploration, the presence of the humans in space and much more). Medicine would not be like it is today if not for fluorine: Many grafts and most anaesthetics rely heavily on fluorine. Coating and surfactants use a lot of it. Humanity is able to provide food to billions of people since it uses agricultural herbicides and insecticides which are very effective because of this element (about 50% of the new agricultural agents will contain it). Today more than 20% of the new drugs responsible for the human well-being incorporate this element (e.g. Lipitor) and we have not said anything yet on the various imaging techniques which rely on fluorine.
It is easy to sum up the subject by remembering that about $300 billion are involved in chemicals containing fluorine, probably more than any other element besides carbon, oxygen and nitrogen.
Researchers:
Emeriti: