A "Super-Cool" Way to Deliver Drugs
TAU researchers discover the secret to substances that can be engineered to "freeze" at a specific time
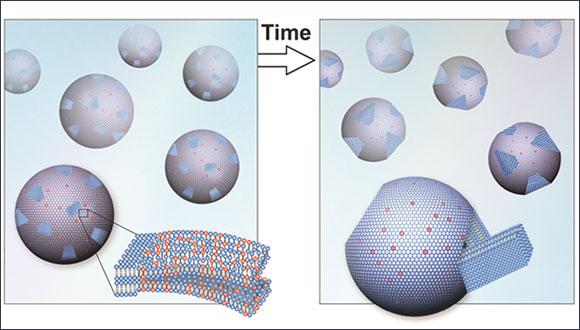
Supercooling describes the process of lowering the temperature of a substance fast enough to "trap" it in its current form, e.g. water at temperatures below 0°C. The phenomenon has been studied for its possible applications in a wide spectrum of fields. A recent publication by Dr. Roy Beck's experimental biophysics group studied the complex process of crystallization in lipid membranes. The study of the nanoscale structures was conducted using a state-of-the-art x-ray scattering system and cryogenic transmission electron microscopy.
The classical nucleation and growth theory describes a supercooled material as 'metastable,' meaning it is very sensitive to any external perturbation that may transform it back to its stable low-temperature state. "What was amazing was our ability to reproduce the results over and over again without any complicated techniques," said Dr. Beck. "We showed that the delayed crystallization was not sensitive to minor imperfection or external perturbation. Moreover, we found multiple alternative ways to 'tweak the clock' and start the crystallization process."
According to the research, it is possible to control what is considered an unpredictable process. Since lipids are used in modern medicine as building blocks for bioengineered drug carriers, this may ultimately aid in the design of the drug delivery systems.
For more information: Scientific Reports 5, 9481 (2015)